How do batteries actually work? Why does a battery warm up if you charge more quickly? In this post, we will explain some basic battery concepts and terminology. This discussion is not comprehensive and is for educational purposes only. For a more in-depth explanation, see a recording from our “Battery Basics” webinar. If you have any ideas for future topics, please submit them on our “Contact Us” page.
Voltage, Current, Resistance:
A battery consists of two electrodes (anode and cathode). The difference in potential between the two determines the battery voltage. During battery operation, the negative electrode is the anode, and the positive electrode is the cathode. Electrons move from the anode to the cathode, negative to positive. Current, however, flows in the opposite direction, from positive to negative.
Current measures the flow of electrical charge (in amps). A battery that discharges at higher current will be used up more quickly than at lower current. Watching a movie on your phone, for example, will drain your battery faster than listening to music - more current is required from the battery.
Resistance is defined as opposition to the flow of current. High resistance in a battery is bad, meaning that current cannot flow properly in and out of the battery. This can cause your battery to heat up during use and “die” much more quickly. There are many causes for resistance to build, including corrosion, loss of electrolyte, temperature effects, and general use over time.
The relationship between voltage, current, and resistance is defined by Ohm’s law, where V = I x R. Assuming constant resistance, the current and voltage are proportional. For example, a circuit has a resistance of 2 Ω and voltage is 24 V. The current can be calculated as 12 A. If we decrease the voltage to 12 V, then the current is now 6 A.
Battery Use:
Many batteries used in electronics and consumer products are rechargeable batteries, that can be discharged and charged multiple times (known as secondary batteries). Batteries designed for one-time use are known as primary batteries. The amount of charge that a battery can hold is defined as amp-hour capacity. A battery with a capacity of 1 amp-hour (Ah) can provide 1 A of current for 1 hour. A battery with 5 Ah capacity is rated to provide 5 A for 1 hour.
Another type of battery rating is the watt-hour energy (Wh): 1 Wh provides 1 watt of power for 1 hour. This term describes the full energy that a battery is able to provide, taking into account the voltage and current. Voltage times current equals work (1V x 1A = 1W). A battery with a rating of 15 Wh can provide 15 W of power for 1 hour. You have probably encountered this unit on your monthly energy bill but depicted in kilowatt-hours (kWh): 1000 Wh = 1 kWh. A driving factor in battery development is packing more energy (Wh) into a smaller format, through different chemistries, formats, or technology.
The term C-rate describes the speed at which a battery is charged or discharged. If you charge at 1C, the battery will go from empty to full in 1 hour. Any C-rate higher than 1C will charge in less than an hour and any C-rate lower than 1 C will take longer than an hour.
For example, say your battery has 5 Ah capacity: 1C is equal to 5 amps. If you charge your battery at 5 amps, it will go from 0% to 100% in 1 hour. If you double the rate to 2C (or 10 amps), the battery will fully charge in 30 minutes. If you charge at C/5 (or 1 amp), it will take 5 hours to charge the battery. The importance of C-rate is that it allows battery manufacturers to compare charging rates/capacities across many different battery types and sizes.
NiMH Battery:
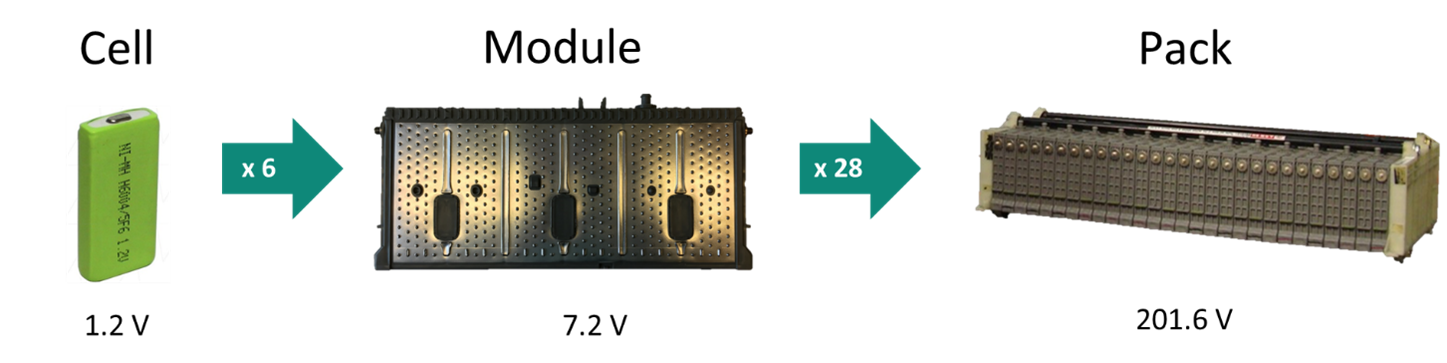
In a NiMH battery cell, the positive electrode contains nickel oxide hydroxide (NiOOH) and the negative electrode contains the metal hydride (MH). The typical voltage of these cells is 1.2 V nominal. The figure below shows the progression of battery cell to battery pack. In a Toyota prismatic module, there are six NiMH cells connected in series, giving you 7.2 V nominal (up to 8.0 V fully charged). The modules are also rated at 6.5 Ah capacity. A Toyota Prius vehicle has 28 of these modules connected in series, giving you 201.6 V and 6.5 Ah. By multiplying the V and Ah, the total Wh energy of the pack is 1310 Wh or 1.31 kWh.
In hybrid electric vehicles, the battery pack assists the internal combustion engine, especially during idling and city driving. HEVs primarily use NiMH batteries, since they do not solely rely on the battery pack for energy, but for better fuel economy. The NiMH battery pack is constantly being charged and discharged during vehicle operation. Features such as regenerative braking maximize efficiency and increase hybrid fuel economy.
Li-ion battery:
There are many different types of lithium-ion batteries, using various chemistries, materials, and formats. The defining characteristic is the use of lithium ions to store energy. The anode usually consists of graphite, while the cathode chemistry can vary. Most common are lithium cobalt oxide (LCO), lithium iron phosphate (LFP), lithium manganese oxide (LMO), lithium nickel manganese cobalt oxide (NMC), and lithium nickel cobalt aluminum oxide (NCA). A primary advantage of Li-ion batteries is higher cell voltage (~3.7 V). This allows for much greater energy density compared to other battery types. Being able to store and deliver large amounts of energy makes them ideal for use in electric vehicles.
A3 Global is a leader in aftermarket HEV and EV batteries. To learn more about our servicing equipment or to purchase remanufactured batteries, please contact us.



